Technologies
-
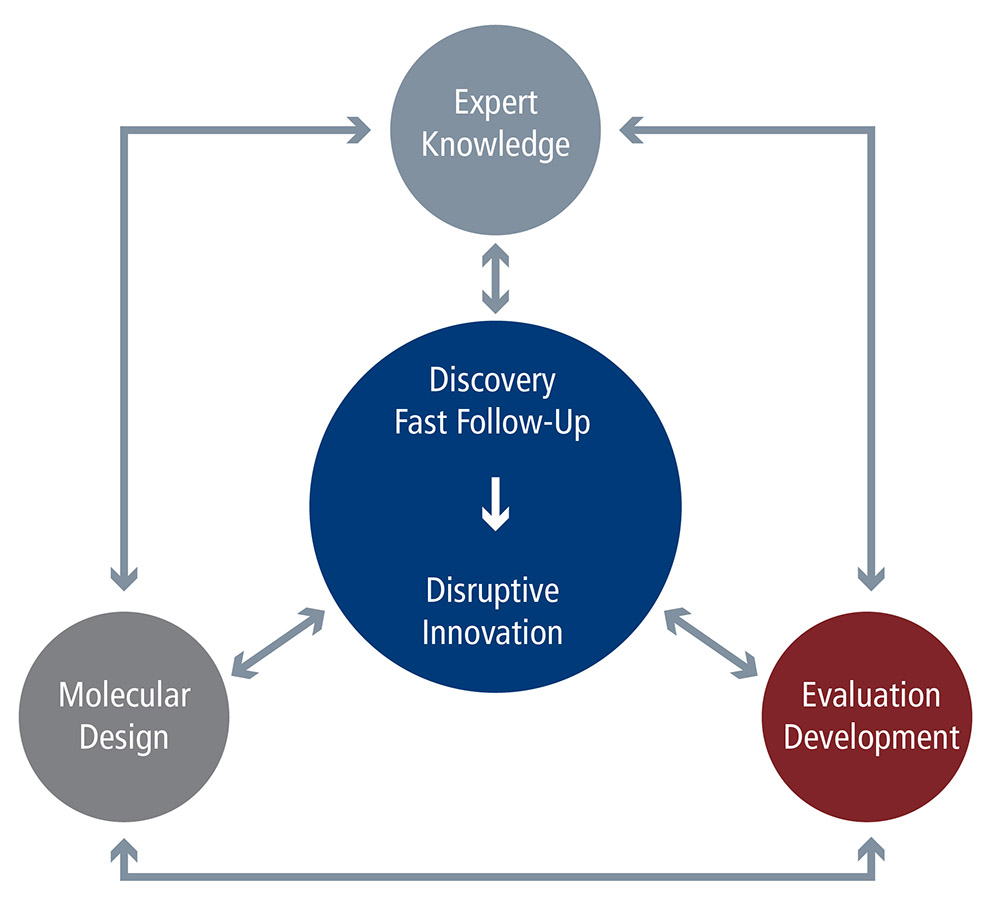
R&D Overview
ChemEOR integrates the latest research and development in oilfield chemistry, materials science, fluid dynamics, surface science, and reservoir engineering to help you make timelier and actionable business decisions, optimize your field operation, and generate greater profitability. Our approach to R&D combines advanced laboratory methods in chemical formulation, monomer design, organic synthesis, and colloid and surface chemistry; with sophisticated computational molecular modeling techniques. This yields a practical product for our clients in a remarkably short lead time. Our commitment to sophisticated yet practical R&D has yielded many established, proven products; as well as the ability to develop variations that address specific customer needs.
UNIQUE R&D APPROACH
Conventional
• Chemist Intuition
• Start with experimental trials
• Empirical approach
• Single experimentOur R&D
• Computer modeling assisted
• Start from molecular design
• Expert/Theory
• High throughput experimentsThis novel approach shortens the development cycle and enables customization to meet customers’ specific requirements.
DOE projects
Academic Publications
SPE Publications
-
Molecular Design
Interdisciplinary
• Chemistry
• Geology
• Geochemistry
• Material Science
• Chemical Engineering
• Petroleum Engineering
• Mechanical Engineering
• Biology
• PhysicsMerits
• Early IP Protection for
molecules identified
• Speed up and reduce costs of
lead identification and
Optimization
• Sustainable technology
advancement
Conventional chemical product development has been based on the guidance from chemical intuition and conventional wisdom, which often results in lengthy laboratory testing based on laborious trial-and-error procedures. In the field, the performance of chemicals in specific applications may vary significantly over time and due to different geological and reservoir environments. Optimizing performance of the chemicals under different reservoir conditions usually requires substantial molecular structural modifications, with respect to which a better fundamental chemistry understanding via theoretical insights can have an important beneficial impact.
 ChemEOR’s unique approach to product development utilizing molecular design combines the experts’ knowledge, novel laboratory evaluation procedures, and advanced computational simulation techniques to obtain quantitative correlations of the molecular structure of chemicals and their performance in different applications. In particular, a series of modern molecular modeling methods, including Quantum Mechanics (QM), Density Functional Theory (DFT), Molecular Dynamics (MD), as well as Quantitative Structure-Activity Relationships (QSAR) and Quantitative Structure-Property Relationships (QSPR) approaches, have been applied routinely to guide our experimental efforts for faster and better product development.
ChemEOR’s unique approach to product development utilizing molecular design combines the experts’ knowledge, novel laboratory evaluation procedures, and advanced computational simulation techniques to obtain quantitative correlations of the molecular structure of chemicals and their performance in different applications. In particular, a series of modern molecular modeling methods, including Quantum Mechanics (QM), Density Functional Theory (DFT), Molecular Dynamics (MD), as well as Quantitative Structure-Activity Relationships (QSAR) and Quantitative Structure-Property Relationships (QSPR) approaches, have been applied routinely to guide our experimental efforts for faster and better product development. -
Fluid Rheology
High molecular-weight water-soluble polymers are frequently added in the aqueous-based fluids to improve their flow and rheological properties, such as increasing viscosity and reducing friction. The fluid dynamics is a sub-discipline of the fluid mechanics that describes the flow of fluids, such as liquids and gases. At ChemEOR, our main interests lie in applications of the fundamental fluid dynamics of liquids, more specifically in the effects of different polymer additives on dynamic and rheological behaviors of fluids, to guide advanced polymer synthesis, manufacture and applications.
Water is widely used as the base fluid in many oilfield applications, such as for drilling, completion, acidizing, stimulation, fracturing, waterflooding, and enhanced production. Polymers, especially the water-soluble polymers, either naturally produced by living organisms or chemically synthesized, have been included in aqueous solutions to improve their flow properties. The fluid dynamic and rheological behavior change with different molecular types (i.e., monomer, charge, and the special functional group), polymer types (i.e., linear, branched, associative and crosslinked), degrees of polymerization and hydrolysis, polymer concentrations, and so on. Therefore, in-depth understanding of the molecular structure of polymer and fluid dynamic theory are equally important in polymer design, manufacture and applications.
Unique Features of ChemEOR’s Technologies
• Molecular-level understanding of polymer physics and fluid rheology for advanced
polymer synthesis, lab and field tests, manufacture and distribution;
• Full-spectrum polymer products manufactured from both biological and chemical
processes, in the form of dry, emulsion, suspension and slurry;
• Custom-designed functionalized products optimized for different oilfield
applications under various reservoir conditions.
One of the primary objectives of ChemEOR is to provide custom-designed high-performance polymer products for a number of oilfield applications, wherein the fundamental polymer science and basic fluid dynamic and rheological theories play important roles in our polymer design, manufacture and applications. A number of important aspects of ChemEOR’s polymer technology are:
• Molecular-level understandings of polymer structures, physical and chemical
properties, such as intra- and inter-molecular forces, H-bonding, polar and non-
polar interactions, self-association and critical association concentration (CAC),
degradation pathways and thermal stability;
• In-depth understanding of the fluid dynamics and rheology of the polymer
solutions, including, non-Newtonian fluid rheology, elasticity, plasticity, viscosity
and polymer concentration, shear rate and stress; and more importantly, the fluid
dynamic and rheological behavior of polymer solutions under high-temperature,
high-pressure and porous rock reservoir conditions;
• Industry-scale manufacture and distribution of high-quality and high-performance
polymer products, such as those commonly used as for fluid thickening, fluid
diverting, gelling, and reducing friction, in forms of dry, suspension, emulsion and
slurry;
• Systematic laboratory experiments and industry-standard testing protocols to
calibrate and evaluate different polymeric products and their chemical
combinations for various oilfield applications.
About Polymer Fluid Rheology
Molecular-level understanding of the polymer structure and the polymer-included fluid dynamic and rheological behaviors are critical to polymer design and applications.Key Ingredients in Polymer Fluid Science
• Density and viscosity
• Critical association
concentration
• Thermal stability and brine
tolerance
• Dispersion, colloidal and
suspension
• Gels, nanoparticles and
particulates
• Laminar and turbulent fluid flow
• Viscous, viscoelastic, and elastic
properties
• Shear rate and stress
-
Surface Chemistry
Surfactants (Surface Active Agents) are usually organic chemicals that have both oil and water soluble components in their structures. A solution containing a surfactant and/or a mixture of surfactants can therefore reduce the oil-water interfacial tension (IFT) (liquid-liquid), or alter the wetting tendency of the reservoir rock surface (liquid-solid), or increase/decrease foaming formation (gas-liquid). Having a fundamental basis of knowledge for the surface chemistry is the key to rationalize surfactant formulation design and selection for various oilfield applications.
Surface Chemistry deals with modification of chemical properties on the interface between two phases, including solid-liquid interface, liquid-liquid interface, liquid-gas interface and solid-gas interface, which are often immiscible or incompatible, wherein surfactants play essential roles. In between of these two phases, there exists a surface tension, or interfacial tension (IFT) that is a more specific term for the liquid-liquid interface, which prevents their mixing together well. A surfactant molecule contains at least two distinguishable parts, each tends to have more affiliation to one side of the interface. When the surfactant is positioned at the interface, it can exhibit unique features to alter its properties.
Unique Features of ChemEOR’s Technologies
• Fundamental theoretical guidance of the surface chemistry to assist surfactant
designs;
• In-depth understanding of surfactant application processes to optimize
performance;
• Advanced chemical engineering to increase surfactant producibility and
sustainability.
With almost an infinite number of potential surfactant candidates, it requires in-depth understanding of the surface chemistry and intelligent designs of the surfactant products to achieve the best product performance in a cost-effective and environmentally-compliant manner. An integrated theoretical and laboratory study guided by conventional expertise and advanced molecule-level understanding of the surface chemistry is essential: ChemEOR’s advanced surfactant technologies include:
• Fundamental understanding of self-assembled surfactant aggregates, such as
micelle, critical micelle concentration (CMC) and packing parameter, displacement,
dispersion, adsorption of surfactants at liquid-liquid interface, at liquid-solid
surface, at gas-liquid or gas-solid phases;
• Application processes of surfactants in emulsion and demulsification,
microemulsion, detergency, surface wetting, foaming, stability, phase and
rheological properties;
• Improving laboratory testing methods to better measure the surface and
interfacial tensions, water-oil emulsion tendencies, surfactant-surface interactions,
and phase behavior of surfactant solutions;
• Improving chemical engineering processes for manufacture of surfactants from
renewable and biodegradable low-cost raw materials.
About Surface Chemistry
Surface chemistry is essential to optimize surfactant formulations for the best performance in a cost-effective and environmentally-compliant manner.Key Ingredients in the Surface Chemistry of Surfactants
• Surfactant types and
functionalities
• Molecular structures of
surfactants
• Emulsion and microemulsion
• Adsorption and absorption
• Foaming and defoaming
• Micelle and packing parameter
• Surface wettability alternation
• Stability and tendency to degrade
• Phase behavior and rheological
properties
-
Commercialization
Molecular Design
Computational simulation techniques and in-depth chemistry fundamentals are combined to predict chemical functionality at molecular-level, significantly shortening the path to high-performance chemical formulations.
Lab Evaluation
Standard industrial testing procedures as well as specifically designed procedures are applied for validation and tuning of our theory, and also for QA/QC of our products.
Performance Materials
Renewable, cost-effective and environmentally-friendly materials are utilized and the applications of advanced bioengineering and nanotechnology methods are also involved.
Customization
The most demanding needs of customers are fulfilled by applying the latest technology to product design and improvement.
Field Application
Optimization and improvements for our products never stop based on the feedback from oilfield application.
• DOE-SBIR 1047290 Phase I Environmentally-Friendly Self-Thickening Chemicals for Improved Conformance Control (01/2011-12/2011)
• DOE RPSEA 07123-2 Performed Particle Gel for Conformance Control (07/2008-03/2011)
• DE-FC26-06NT15525 Bio-Engineering High Performance Microbial Strains for MEOR by Directed Protein Evolution Technology (10/2004-09/2007)
• DE-FC26-04NT15521 Cost Effective Surfactant Formulations for Improved Oil Recovery in Carbonate Reservoirs (10/2004-03/2007)
• DE-FC26-01BC15362 Lower Cost Methods for Improved Oil Recovery (IOR) via Surfactant Flooding (09/2001-09/2004)
• SPE 179667 Surfactant Huff-n-Puff Application Potentials for Unconventional Reservoirs. (2016)
• SPE 179525 Possibility of Flooding Polymer or Water Reuse via Innovative Selective or Total Flocculation of Enhanced Oil Recovery Produced Water (2016)
• SPE 178992 Results from Response Surface Methodology in the Study of Novel Temporary and Permanent Clay Stabilizers (2016)
• SPE 173753 Computational Modeling of Temporary Clay Stabilizers Supported by Performance Testing (2015)
• OTC-25869-MS Effects of BETX on the Abnormal Geopressures (2015)
• SPE 171025 Friction Reducers Fresh Rheological Insights Married to Performance (2014)
• SPE 168180 Optimizing Surfactant Additives for Enhanced Well Stimulation in Bakken Formation. (2014)
• CSUG/SPE 147531 Chemical Process for Improved Oil Recovery From Bakken Shale (2011)
• SPE 143514 Research on a New Profile Control Agent: Dispersed Particle Gel (2011)
• SPE 132564 Heavy Oil Production Enhancement by Viscosity Reduction (2010)
• SPE 124257 A New Method for Fast Screening of Long Term Thermal Stability of Water-Soluble Polymer for Reservoir Conformance Control (2009)
• SPE 99612 An Experimental Study of Wetting Behavior and Surfactant EOR in Carbonates with Model Compounds (2008)
• SPE 106048 Engineering Rhamnolipid Biosurfactants as Agents for Microbial Enhanced Oil Recovery (2007).
• SPE 95404 A Study of Branched Alcohol Propoxylate Sulfate Surfactants for Improved Oil Recovery (2005).
• SPE 89472 Alkyl Polyglycoside Surfactants for Improved Oil Recovery (2004).
• SPE 80243 Improved Transportation of Waxy Crude Oils and Emulsions in Bekasap Area, Indonesia. (2003)
• Evaluation of Functionalized Polymeric Surfactants for EOR Applications in the Illinois Basin, Journal of Petroleum Science and Engineering. 2015, 134, 167-175
• Chemical and Thermal Stability of N-heterocyclic Ionic Liquids in Catalytic C-H Activation Reactions, Magnetic Resonance in Chemistry, 2014, 52, 673-679
• Dilute iota- and kappa-Carrageenan solutions with high viscosities in high salinity brines. Journal of Petroleum Science and Engineering. 2011, 75, 304-311
• Alkyl polyglycoside surfactant-alcohol cosolvent formulations for improved oil recovery. Tenside Surfactants Detergents. 2010, 47, 48-59
• New surfactant classes for enhanced oil recovery and their tertiary oil recovery potential. Journal of Petroleum Science and Engineering. 2010, 71, 23-29
• Analysis of the Influence of Alkyl Polyglycoside Surfactant and Cosolvent Structure on Interfacial Tension in Aqueous Formulations versus n-Octane. Tenside Surfactants Detergents. 2010, 47, 87-97
• Alkyl Polyglycoside Surfactant-Alcohol Cosolvent Formulations for Improved Oil Recovery. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 2009, 339, 48-59
• Engineering bacteria for production of rhamnolipid as an agent for enhanced oil recovery. Biotechnology and bioengineering. 2007, 98, 842-853
• Molecular Dynamics Study of a Surfactant-Mediated Decane-Water Interface: Effect of Molecular Architecture of Alkyl Benzene Sulfonate. The Journal of Physical Chemistry, B. 2004, 108, 12130-12140
• Evaluation of Effects of Selected Wax Inhibitors on Wax Appearance and Disappearance Temperatures. Petroleum Science and Technology. 2003, 21, 359-368
• Evaluation of Effects of Selected Wax Inhibitors on Paraffin Deposition. Petroleum Science and Technology, 2003, 21, 369-379.
• Measurement of wax deposition in paraffin solutions. AiChE Journal. 2002, 48, 2107-2110
• The MSXX Force Field for the Barium Sulfate-Water Interface. The Journal of Physical Chemistry, B. 2002, 106, 9951-9966
• Scanning Force Microscopy Study of Etch Pits Formed during Dissolution of a Barite (001) Surface in CDTA and EDTA Solutions. Langmuir, 2000, 16, 649-655.
• Dissolution of the barite (001) surface by the chelating agent DTPA as studied with non-contact atomic force microscopy. Colloids and Surfaces A: Physicochemical and Engineering Aspects. 1999, 160, 217-227
• Study of the Dissolution of the Barium Sulfate (001) Surface with Hydrochloric Acid by Atomic Force Microscopy. Journal of Colloid and Interface Science. 1999, 219, 212–215
• Atomistic Simulations of Oleic Imidazolines Bound to Ferric Clusters. The Journal of Physical Chemistry, A, 1997, 101, 83-89
• Self-Assembled Monolayer Mechanism for Corrosion Inhibition of Iron by Imidazolines. Langmuir, 1996, 12, 6419-6428